July 6, 2018 — Dr. Keith Ayoob, Associate Clinical Professor Emeritus at the Albert Einstein College of Medicine in New York, spoke to delegates at the International Union of Nutritional Sciences (IUNS) International Congress of Nutrition (ICN), held in Buenos Aires, Argentina October 15-20, 2017. In his presentation, he discussed two of today’s most common health epidemics – obesity and Type 2 diabetes – and reviewed the science confirming the role low and no calorie sweeteners (LNCS) such as stevia can play in reducing added sugar intake. Dr. Ayoob’s presentation “Health and Wellness of Stevia as a Sweetener” drew from in-vitro, animal and human studies, and discussed stevia’s potential role in helping manage diabetes, blood pressure, weight, and appetite.
July 6, 2018 — Dr. Keith Ayoob, Associate Clinical Professor Emeritus at the Albert Einstein College of Medicine in New York, spoke to delegates at the International Union of Nutritional Sciences (IUNS) International Congress of Nutrition (ICN), held in Buenos Aires, Argentina October 15-20, 2017. In his presentation, he discussed two of today’s most common health epidemics – obesity and Type 2 diabetes – and reviewed the science confirming the role low and no calorie sweeteners (LNCS) such as stevia can play in reducing added sugar intake. Dr. Ayoob’s presentation “Health and Wellness of Stevia as a Sweetener” drew from in-vitro, animal and human studies, and discussed stevia’s potential role in helping manage diabetes, blood pressure, weight, and appetite.
Understanding its Role in the Fight Against Obesity and Diabetes
BY: Dr. Keith Ayoob
Key Objectives:
- Explore the extent of the global health epidemics of obesity and type 2 diabetes.
- Discuss specifics of global health recommendations to reduce intake of added sugars and the role played by zero-calorie sweeteners in achieving these recommendations
- Explore the unique role plant-based stevia may have in helping persons with diabetes manage blood glucose levels.
- Learn and apply information related to the benefits of natural-origin stevia and the opportunities and challenges in developing reduced-calorie-reduced-sugar foods with sweeteners/stevia
Global Obesity Epidemic
The issue of global obesity is worsening. The International Diabetes Foundation predicts that 641 million people will have diabetes by 2040, up from 415 million in 2015. In North America, the prediction numbers 60.5 million by 2040, compared with 44.3 million in 2015. Obesity is a gateway disease for diabetes, which in turn is a gateway disease for chronic conditions such as metabolic syndrome, hypertension, cardiovascular risk, retinopathy, and more.
Worldwide authorities have made a call for healthier lifestyles including reductions in the consumption of total calories and especially added sugar. The World Health Organization (WHO) recommends a decrease in added sugars to less than 10% of total calories, with the organization ultimately aiming for less than 5% of total calories sourced from added sugars. Without changes, such as replacing sugar with LNCS, this level of reduction will be extremely difficult for most people to achieve in the present environment.
In reducing calories from sugar, and in looking at low calorie sweetener options, let’s look at the scientific evidence and efficacy of stevia as an option in sugar reduction.
Scientific Evidence of Stevia
from [TT1] Evidence Based Systematic Reviews and Meta Analyses
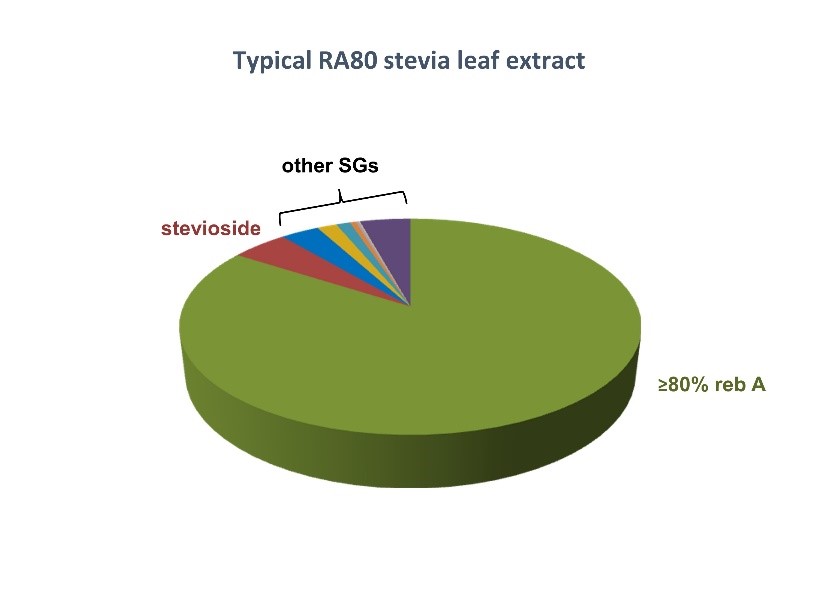
The European Food Safety Authority (EFSA)[1] reviewed studies on steviol glycosides (a sweet component from stevia leaf extract):
- In-vitro studies showed a stimulation in insulin secretion from islet cells, up-regulation of key genes controlling insulin secretion and a positive impact on insulin signaling and release.
- Animal studies indicated an improved insulin sensitivity and plasma glucose levels in normal, type 2 diabetic or obese rats as well as a decrease in blood glucose levels, possibly by enhancing insulin secretion and regulating gluconeogenesis.
- Human studies in persons with and without type 2 diabetes, up to 1000 mg preparation, showed no negative effect on glucose homeostasis, with some studies showing reduced postprandial glucose in persons with diabetes. In addition, there was no effect on blood pressure in either those with or without type 2 diabetes.
In the Onakpoya and Heneghan, et el[2] meta-analysis of nine human randomized controlled trials (RCT) studies of various pharmacologic doses, stevioside showed no dose-response relationship between stevia and cardiovascular factors, and in particular showed a small reduction in blood pressure.
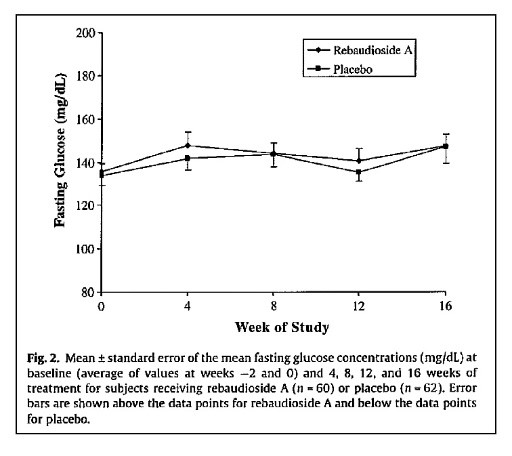
In the Maki KC, et el[3] 16-week study , 122 diabetic adults were given 1000mg of Reb A stevia per day. The study found there to be no negative effects on glycemic load, HbA1C, fasting glucose, or serum insulin, noting that the results held even at a pharmacologic dose.
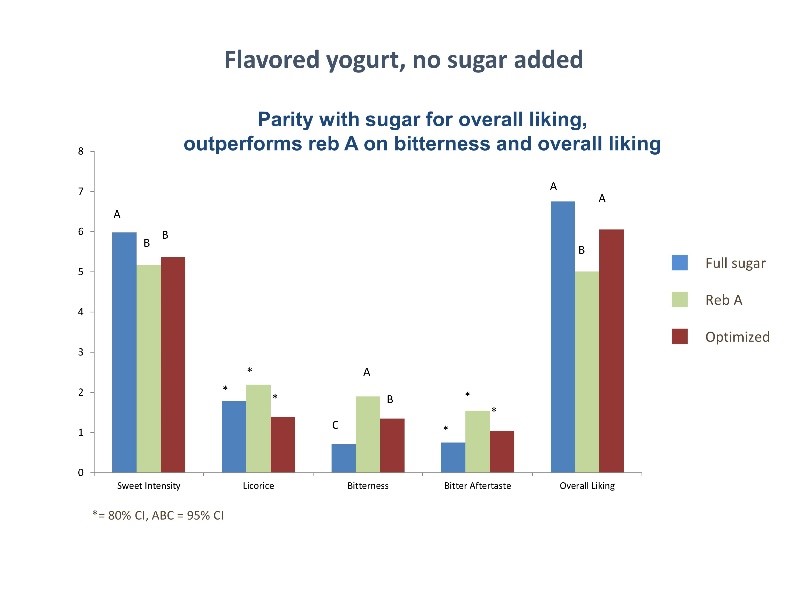
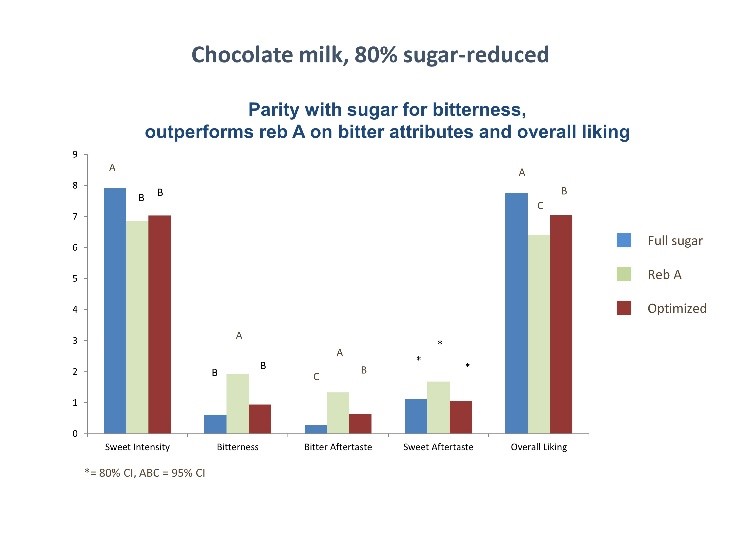
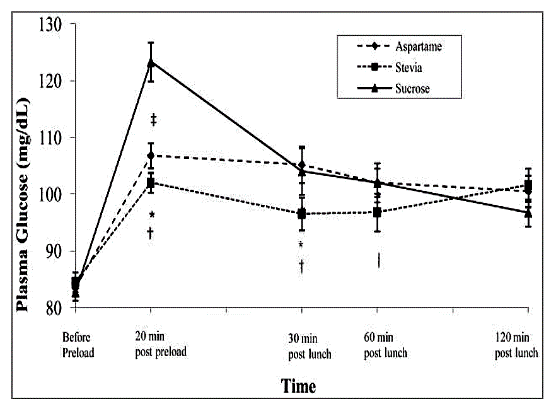
The Mohd-Radzman, et el[4] review on the potential role of stevia in managing insulin resistance and diabetes in animal studies indicated a decrease in lipid peroxidation when pre-fed with stevia and an increase in insulin secretion, suggesting slower or reduced progression of diabetic co-morbid complications. In human studies, there was a decrease in postprandial glucose levels when fed meals supplemented with stevioside, compared with both sucrose and aspartame. Researchers further noted that stevia seems to have a target-specific effect, by reducing hyperglycemia in human subjects (1gm dose), while having no effect in normoglycemic conditions, suggesting no danger of hypoglycemia. (Evidence Based Com Alt Med)
Efficacy of Stevia
in [TT2] Real Life
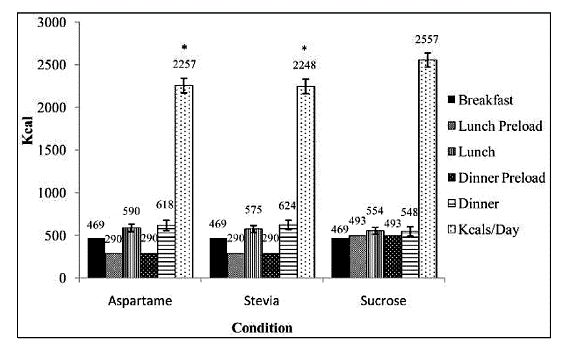
A human study conducted by Anton SD, et al[5] showed less postprandial glucose spiking with stevia in a reduced-calorie meal, compared with a sucrose-laden meal, and with no differences in hunger or satiety from the sucrose group.
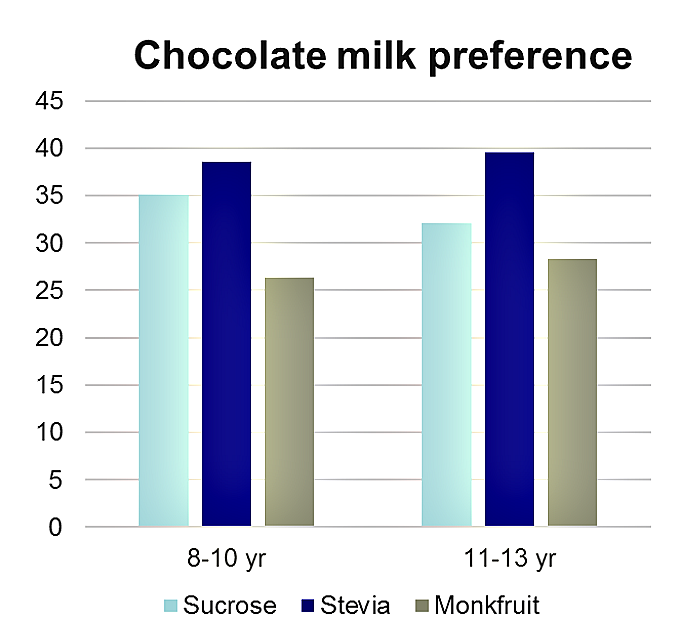
In a human study conducted by Li, et al[6] with individuals 8-13 years of age, children actually preferred the stevia-sweetened chocolate milk, and that “label-conscious” parents preferred seeing stevia on the label rather than sugar, whereas the “traditional” parents preferred the label indicating the sugar-sweetened milk.
An Expert Consensus Statement from Gibson, et al[7] showed agreement that low- and no- calorie sweeteners can be useful tools for replacing high-calorie ingredients, enhancing weight loss efforts, managing postprandial glucose and insulin levels in both persons with and without diabetes without changes in appetite or satiety, and providing dental health benefits.
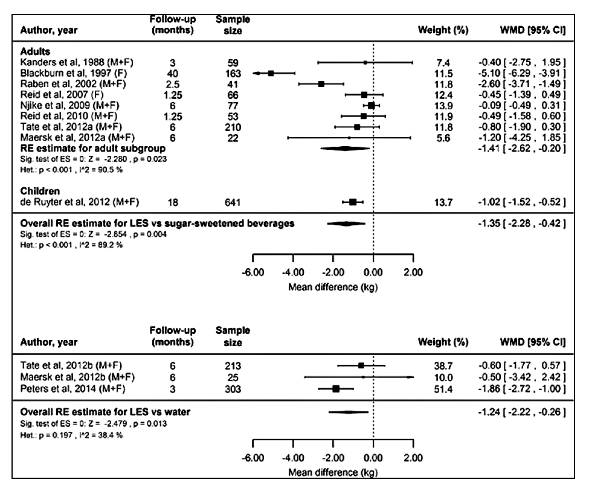
The Miller, et al[8] meta-analysis of 15 RCTs and 9 prospective cohort studies showed significant body weight reductions from the use of LNCS, versus sugar sweetened beverages and even water, with the analysis concluding that the LNCS were useful tools for improving compliance with weight loss and weight maintenance efforts.
The Rogers PJ et al[9] meta-analysis also noted that low calorie sweeteners do not increase energy intake or body weight, whether compared with caloric or non-caloric conditions.
Take-Aways
Weight Management
Foods containing stevia may help with a long-term modest effect on body weight, body mass index (BMI) and waist circumference.
Appetite
Foods containing stevia help lower total calorie intake, without over-consumption later in the day.
Diabetes
Stevia has been confirmed as safe and appropriate for persons with diabetes. As a sugar replacer, stevia may benefit blood glucose & insulin levels, with no negative effect on glucose homeostasis.
Blood Pressure
Long-term, the use of stevioside may have a small lowering effect on blood pressure, though most studies used consumption levels higher than the acceptable daily intake (ADI).
In Conclusion
When substituted for sugar, stevia can help with weight management by reducing added sugar and calories. Stevia can be used by anyone, including normal-weight persons, who simply want to reduce overall sugar intake and improve dietary quality. All major regulatory bodies found stevia to be safe and suitable for the entire family.
One Caveat: Stevia, like all LNCS, is a tool for managing weight and dietary quality, but should not be the only tool. Placing the burden of solving the obesity crisis on a single factor would be inappropriate. This requires a gradual change in eating style, lifestyle, with stevia and LNCS as part of that plan.
For a copy of the complete presentation, click here.
For more information about stevia, contact the International Stevia Council or the Calorie Control Council.
About Dr. Keith Ayoob
 Keith Ayoob, EdD, RDN, FADN is an Associate Clinical Professor Emeritus at the Albert Einstein College of Medicine. As a pediatric nutritionist and registered dietitian, Dr. Ayoob is also a past national spokesperson for the Academy of Nutrition and Dietetics.
Keith Ayoob, EdD, RDN, FADN is an Associate Clinical Professor Emeritus at the Albert Einstein College of Medicine. As a pediatric nutritionist and registered dietitian, Dr. Ayoob is also a past national spokesperson for the Academy of Nutrition and Dietetics.
Dr. Ayoob is a consultant with the Calorie Control Council Advisory Board and the Global Stevia Institute (GSI), GSI is supported by PureCircle Ltd, a global leader in purified stevia leaf extract production.
1 EFSA. 2010a. Scientific opinion on the safety of steviol glycosides for the proposed uses as food additive. EFSA J 8(4):1537 [85 pp.]; doi: 10.2903/j.efsa.2010.1537. Available from: http://www.efsa.europa.eu/en/ efsajournal/doc/1537.pdf.
2 Onakpoya IJ, Heneghan CJ. Effect of the natural sweetener, steviol glycoside, on cardiovascular risk factors: a systematic review and metaanalysis of randomised clinical trials. Eur J Prev Card. 2015;22:1575–87
3 Maki, K., Curry, L., Reeves, M., Toth, P., Mckenney, J., Farmer, M., et al. (2008). Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food and Chemical Toxicology, 46, 47–53.
4 N. H. Mohd-Radzman, W. I. W. Ismail, Z. Adam, S. S. Jaapar, and A. Adam, “Potential roles of Stevia rebaudiana Bertoni in abrogating insulin resistance and diabetes: A Review,” Evidence based Complementary and Alternative Medicine, vol. 2013, Article ID 718049, 10 pages, 2013.
5 Anton, S., Martin, C., Han, H., Coulon, S., Cefalu, W., Geiselman, P., et al. (2010). Effects of Stevia, aspartame, and sucrose on food intake, satiety and postprandial glucose and insulin levels. Appetite, 55, 37–43.
6 Li XE, Lopetcharat K, Drake MA. Parents’ and children’s acceptance of skim chocolate milks sweetened by monk fruit and stevia leaf extracts. J Food Sci. 2015;80:S1083-92.
7 Gibson S, Drewnowski A, Hill J et al. Consensus statement on benefits of low-calorie sweeteners. Nutrition Bulletin 2014; 39(4): 386–9.
8 Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014; 100: 765-777.
9 Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes 2016; 40: 381-394
©Copyright 2017 – 2018



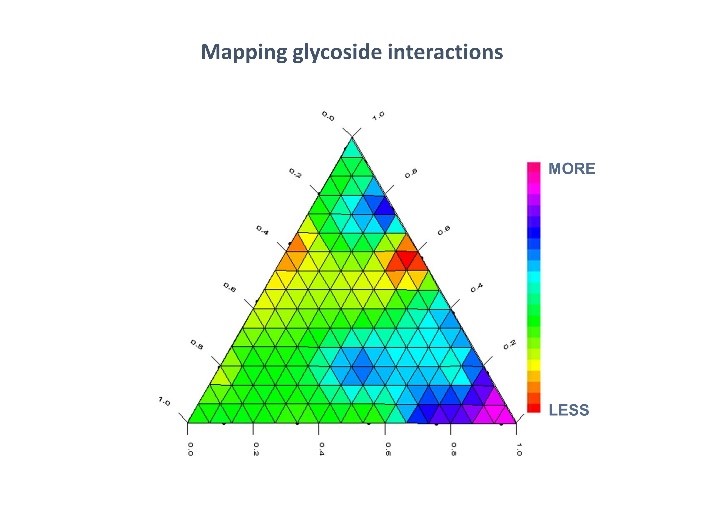
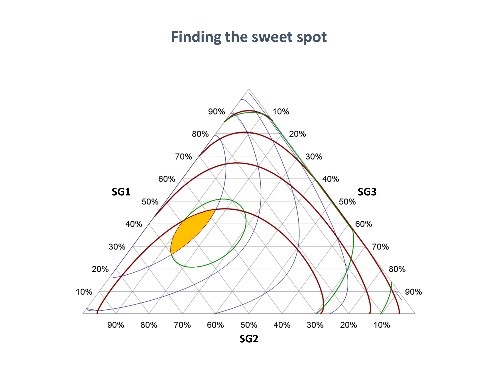
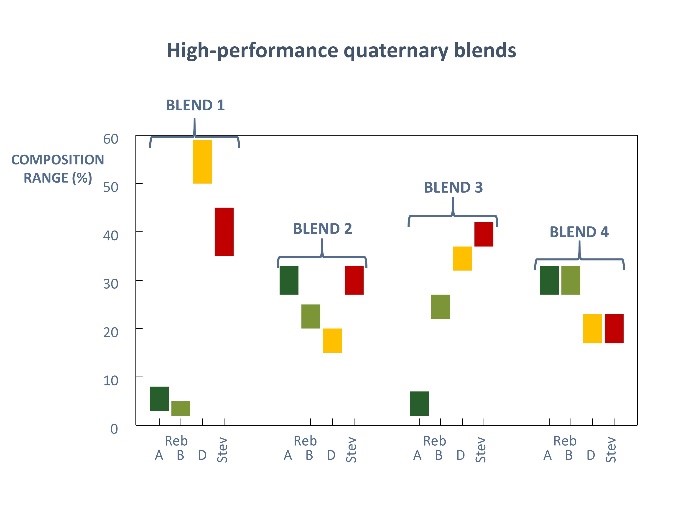
 June 28, 2018 — Dr. John Fry, internationally-acknowledged expert on high-potency sweeteners, presented “Application and Innovation in Stevia and Taste Development: Improved leaf extracts from advanced sensory study” at the International Union of Nutritional Sciences (IUNS) International Congress of Nutrition (ICN), held in Buenos Aires, Argentina October 15-20, 2017. Discoveries he described are now helping to create today’s stevia sweeteners with greatly improved taste. Read on as Dr. Fry explains important research findings, in addition to more on advances made in the taste profile of one of the fastest growing sweeteners today.
June 28, 2018 — Dr. John Fry, internationally-acknowledged expert on high-potency sweeteners, presented “Application and Innovation in Stevia and Taste Development: Improved leaf extracts from advanced sensory study” at the International Union of Nutritional Sciences (IUNS) International Congress of Nutrition (ICN), held in Buenos Aires, Argentina October 15-20, 2017. Discoveries he described are now helping to create today’s stevia sweeteners with greatly improved taste. Read on as Dr. Fry explains important research findings, in addition to more on advances made in the taste profile of one of the fastest growing sweeteners today.